Studies have shown that there is a significant association between occupational exposure during a normal workday and increases in chromosomal aberration in healthcare workers. It is essential to comply with USP <800> in order to protect the patients and staff exposed to hazardous drugs in your facilities.
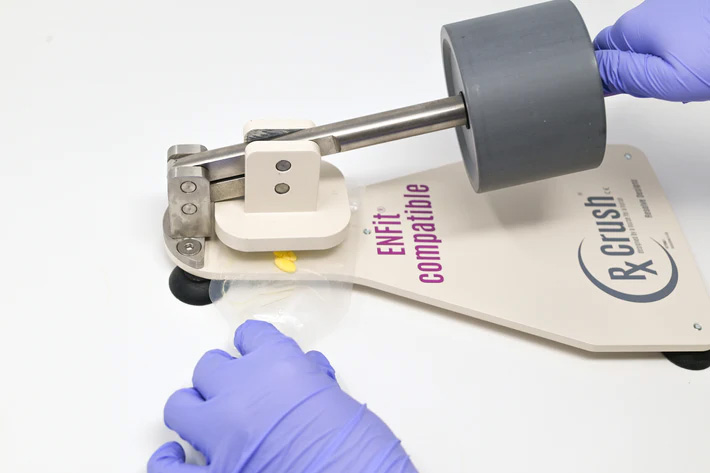
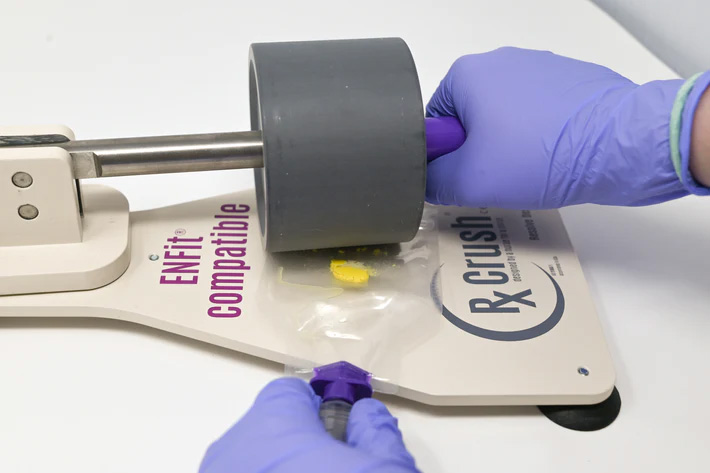
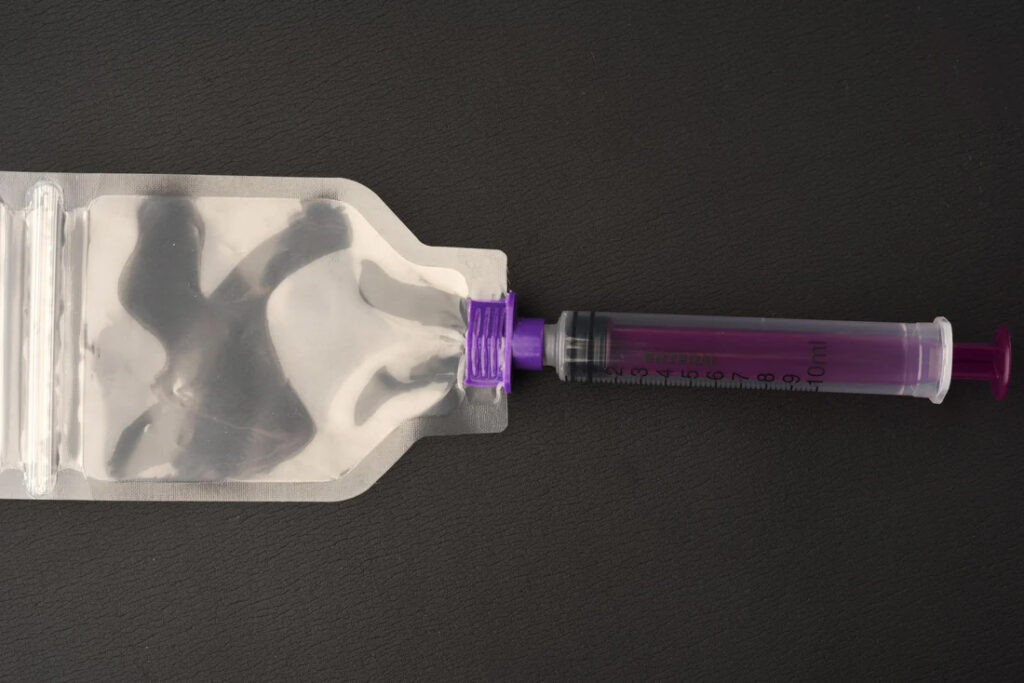
Dust from tablets and capsules may present a risk of exposure by skin contact and/or inhalation. If HD dosage forms do require manipulation such as crushing tablet(s) or opening capsule(s) for a single dose, personnel must don appropriate PPE and use a plastic pouch to contain any dust or particles generated.
USP 800 Hazardous Drugs – Handling in Healthcare Settings was developed to provide new quality standards for handling Hazardous Drugs in all settings, with an end goal of ensuring the safety of both patients and staff who come in contact with HDs.
USP 800 will be enforced at the federal level primarily by the Occupational Safety and Health Administration (“OSHA”), and at the state level by state boards of pharmacy.